The World Health Organization is warning the public to stay away from counterfeit Ozempic, or “fauxzempic.” The agency has recently received reports of medication falsified to appear like the diabetes and off-label weight loss drug Ozempic in at least three countries, including the U.S.
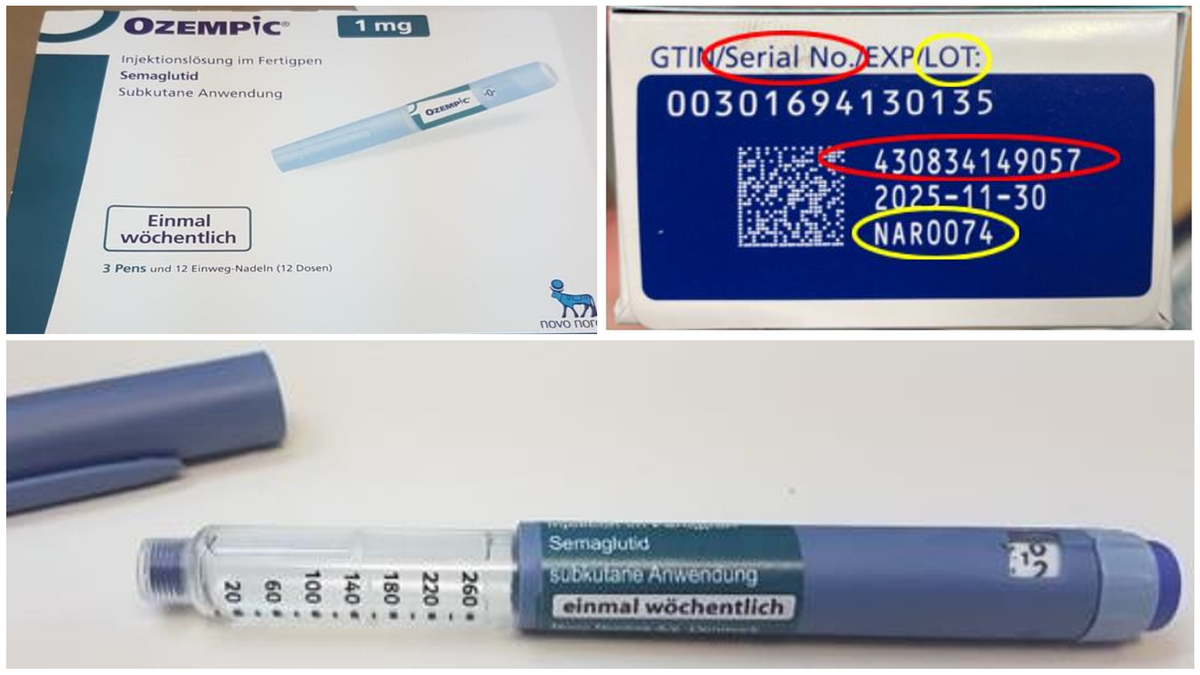
The WHO issued a medical product alert about the fake medication Wednesday, along with a public announcement Thursday. The alert concerns three falsified batches discovered in three countries. Two batches were detected last October in Brazil and the UK, respectively, while a third batch was uncovered in the U.S. last December. All three were reportedly confirmed as fraudulent by Ozempic’s manufacturer, Novo Nordisk.
In one lot, the product was labeled with a falsified batch number. In another lot, the combination of batch and serial numbers did not match official records. And in the third, the batch number was accurate, but the actual product was falsified.
“WHO advises healthcare professionals, regulatory authorities and the public be aware of these falsified batches of medicines,” said Yukiko Nakatani, WHO Assistant Director-General for Access to Medicines and Health Products, in a statement from the agency. “We call on stakeholders to stop any usage of suspicious medicines and report to relevant authorities.”
The active ingredient in Ozempic is semaglutide, which is part of a class of drugs known as incretins. Incretins interact with or mimic hormones that regulate our sense of hunger and blood sugar, among other functions, with semaglutide being a mimic of GLP-1. In clinical trials, semaglutide and other newer incretins have proven to be much more effective at treating obesity than diet and exercise alone. Ozempic is only approved for type 2 diabetes, but a separate higher-dose version of semaglutide was approved for obesity under the name Wegovy in 2021. Following Wegovy’s approval, Ozempic has become routinely prescribed off-label for weight loss as well.
Unfortunately, the demand for these drugs has often overwhelmed the supply. These shortages, combined with high list prices (upwards of $1,000 a month) and low insurance coverage, has led to a gray and black market for the drugs.
Some people are obtaining their GLP-1s from compounded pharmacies, which are typically used to create custom-made drugs for patients with special needs, such as drugs free of certain allergens. These pharmacies serve a legitimate purpose and are supposed to source their active ingredients from regulated facilities, but many places selling compounded semaglutide are doing so illegally, according to the National Association of Boards of Pharmacy. Regulators have also begun to come across counterfeit Ozempic made to look like the real deal. In at least some cases, the products actually contained insulin and users have been hospitalized with low blood sugar and seizures as a result of overdosing on it.
While these compounded and counterfeit medications do tend to be much cheaper than the official version, at least without insurance coverage, they are riskier to take, given the lack of regulation and oversight. The WHO is advising people who need these drugs to obtain them from licensed physicians and to avoid buying them from unfamiliar places, particularly online pharmacies.
“The use of falsified OZEMPIC may result in the ineffective treatment of patients due to incorrect dosage, contamination with harmful substances, or use of unknown or substituted ingredients. It may pose other serious risks to health because of its subcutaneous injection administration that could be life-threatening,” the WHO noted in its alert.




